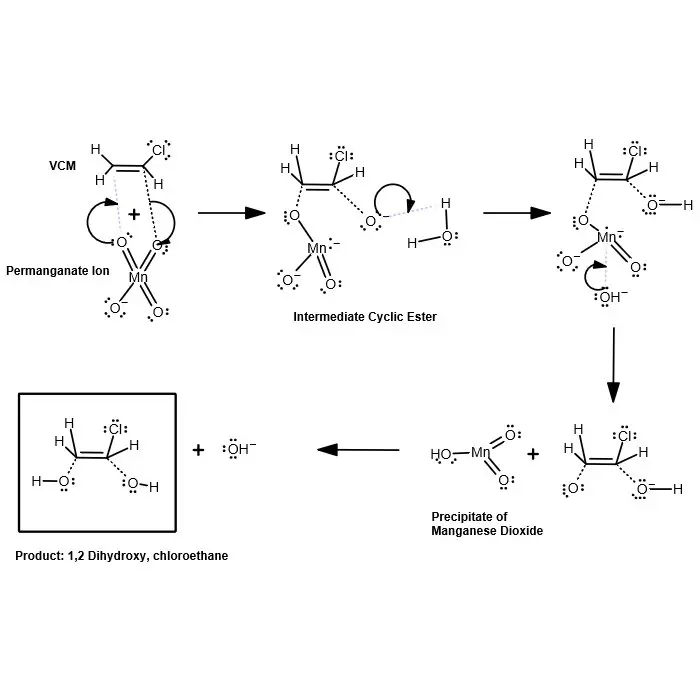
Reaction For Removal of Vinyl Chloride Using Potassium Permanganate
The reaction of the permanganate ion with vinyl chloride monomer is outlined in the figure above. The reaction produces 1,2 dihydroxy, chloroethane, an addition product, and a precipitate of manganese dioxide. A short description of the reaction is also included below. The typical oxidation reaction for an alkene by permanganate ion may be found in any general organic chemistry text.
The oxidation of an alkene leads to the formation of a compound with hydroxyl groups on the carbon atoms that were involved in the double bond, a 1,2 diol. Manganese (VII) in permanganate ion is ultimately reduced to manganese (IV) in manganaese dioxide. The carbon atoms of the double bond are oxidized. Even if no base is added at first, the solution becomes progressively more basic as the reaction proceeds.
In this oxidation reaction, the two hydroxyl groups become attached to the same face of the double bonds. The permanganate ion is believed to add to the double bond to give a cyclic intermediate a manganate ester. The first step of this reaction is the syn (same side) addition of permanganate ion to the double bond. This intermediate breaks down in the presence of water to give the cis-1,2 diol. Thus, there are no appreciable quantities of chlorine gas or formaldehyde formed in the reaction.